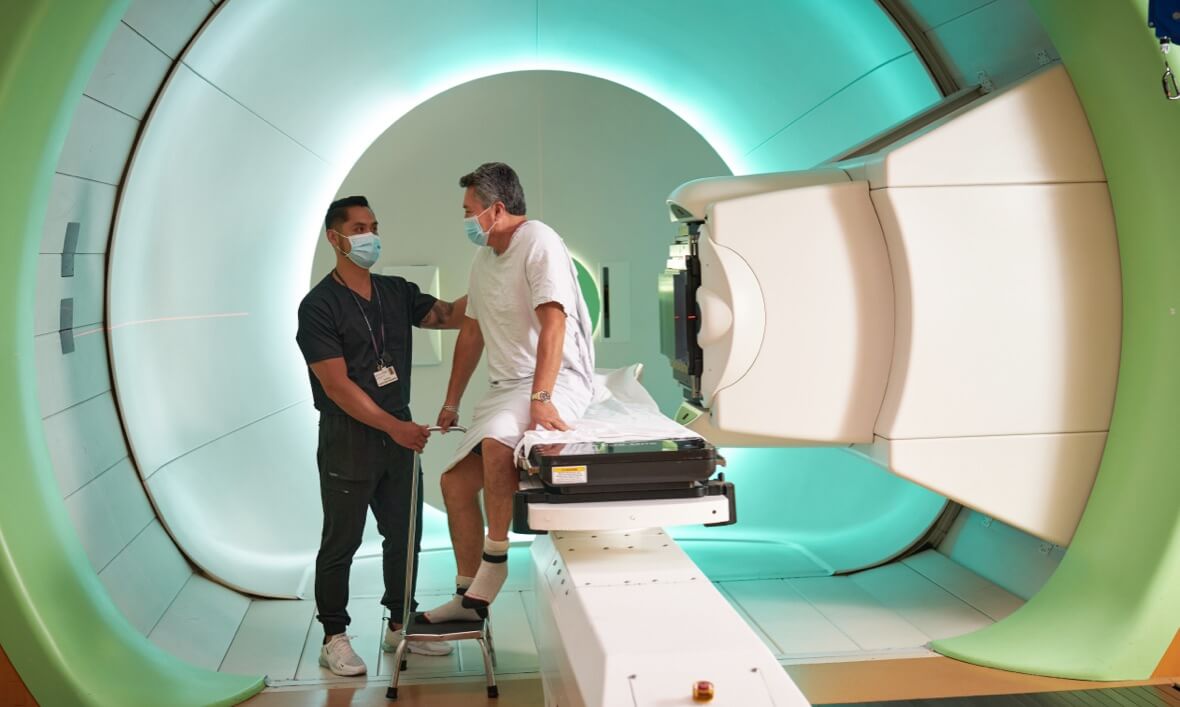
With five treatment rooms and a dedicated research lab, the center operates as the world’s largest hub for the integration of proton therapy and conventional radiation therapy. Every member of the center’s team has specialized training and depth of experience in proton beam therapy, a type of radiation therapy whose high-energy radiation beams offer greater precision and accuracy in destroying cancer cells than that of conventional therapies.
Thanks to the center’s unique research-and-care infrastructure—where scientists can use photon and proton radiation side by side, and within feet from the clinic—researchers are developing an experimental therapy known as FLASH radiotherapy.
Imagine cancer patients being able to receive an entire course of radiotherapy in a single rapid treatment rather than over the duration of weeks and months. That’s FLASH radiotherapy, and according to the Abramson Cancer Center’s research (the results of which were recently published in the International Journal of Radiation Oncology, Biology and Physics), it offers hope for the future of oncology.
"For the first time, we can demonstrate that using proton radiation to generate FLASH radiotherapy is feasible," says the study’s co-senior author, James M. Metz, MD, Henry K. Pancoast Professor and Chair of the Department of Radiation Oncology.
Other advances in proton therapy at Penn include pencil beam scanning, in which the proton beam is mere millimeters wide and can be adjusted in intensity to deliver more radiation to targeted areas; and advanced imaging technology integrated into the proton therapy machines to calculate the most accurate radiation doses possible.
"This research is high-risk, high-reward, very early stage and not typically fundable through most methods," says Andy J. Minn, MD, PhD, Associate Professor of Radiation Oncology. "It really takes partnerships with funders and an academic center like Penn to make these types of ideas a reality."